When you buy a supplement the first thing you should look for is QUALITY. Quality is the #1 concern you should have because it has everything to do whether:
a) you’re getting what is inside the bottle which is on the label
b) you’re getting a supplement that is the most effective it can be. (that is works essentially)
c) you’re not taking something worthless, dangerous, or mis-branded.
Quality is very, very hard to determine. Supplement companies marketing is so crafty that it takes a true supplement expert to know whether a supplement is high quality or not. So many supplement companies claim to be experts in making supplements, selling supplements, formulating supplements, etc, etc. However this is far from the truth. The truth of the matter is most supplement companies BULLSHIT their way in manufacturing supplements and try to make their potential customers THINK they are experts by using smoke and mirrors. REAL supplement manufacturing experts like myself KNOW how to spot scammers and wanna-be’s. And you, the consumer needs to be aware of who’s using smoke and mirrors to pretend their supplements are PHARMACUETICAL GRADE!! (that’s bullshit BTW, I was being sarcastic). One way to clear the smoke and mirrors is to analyze their supplement labels. When we analyze the labels and see numerous FDA violations, it gives us a good indication that the supplement company whom product has all these violations on their label isnt really the experts they think they are.
Every week I’m going to try point out supplement companies labels that we feel are in violation with the FDA regulations. When you are violating the regulations the FDA deems the product MIS-BRANDED. When your product is mis-branded it needs to be fixed because it is violating the law. If it is not fixed the FDA can force the product off the retail shelf. Because the FDA is so short-handed, I’m going to help them out and point out supplements that are (in our opinion) mis-branded.
I also will be emailing these labels to the FDA. (hey, I’m just trying to help)
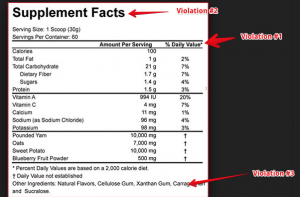
The following label is a supplement called REAL FOOD. Kinda strange to call it REAL FOOD, when it has a supplement facts panel. Which means its a supplement, not a food. I dont think the FDA would like this…but that’s besides the point. Anyway, lets get to their violations. According to the opininon of my supplement experts that we employee here at Proteinfactory.com. This label has 3 violations.
VIOLATION #1
The * notating the %DV should be placed next to each value entered, not the top of the column as it is listed.
21 cfr 101.36(D) If the percent of Daily Value is declared for total fat, saturated fat, total carbohydrate, dietary fiber, or protein, a symbol shall follow the value listed for those nutrients that refers to the same symbol that is placed at the bottom of the nutrition label, below the bar required under paragraph (e)(6) of this section and inside the box, that is followed by the statement “Percent Daily Values are based on a 2,000 calorie diet.”
VIOLATION #2
What is wrong: the supplement facts heading does not span the width of the supplement facts panel
21 cfr 101.36(e)(1) The title, “Supplement Facts,” shall be set in a type size larger than all other print size in the nutrition label and, unless impractical, shall be set full width of the nutrition label. The title and all headings shall be bolded to distinguish them from other information
Violation #3
What is wrong: The format of the panel as it stands, is not compliant. The hairline and heavy bars are not set as required. The other ingredients disclosure, needs to be moved outside of the panel.
21 cfr 101.36 (e)(5) A hairline rule that is centered between the lines of text shall separate each dietary ingredient required in paragraph (b)(2) and (b)(3) of this section from the dietary ingredient above and beneath it, as shown in paragraph (e)(10) of this section.
21 cfr 101.36(e)(6) A heavy bar shall be placed:
(i) Beneath the subheading “Servings Per Container” except that if “Servings Per Container” is not required and, as a result, not declared, the bar shall be placed beneath the subheading “Serving Size,”
(ii) Beneath the last dietary ingredient to be listed under paragraph (b)(2)(i) of this section, if any, and
(iii) Beneath the last other dietary ingredient to be listed under paragraph (b)(3) of this section, if any.
(7) A light bar shall be placed beneath the headings “Amount Per Serving” and “% Daily Value.”
This label in our opininon is mis-branded and an FDA inspection of this company should yield a 483 warning letter. This means the company and their product REAL FOOD is violating the CFR 111’s.
And no, the supplement companies manufacturer is NOT responsible for the label and the product. The owner of this supplement company is.
Alex Rogers is a supplement manufacturing expert. He has been formulating, consulting, & manufacturing dietary supplements since 1998. Alex invented protein customization in 1998 & was the first company to allow consumers to create their own protein blends. He helped create the first supplement to contain natural follistatin, invented whey protein with egg lecithin, & recently imported the world’s first 100% hydrolyzed whey.