If you are a supplement company and find your name on this list read this first before threatening to sue me. Because if you threaten to sue me, you’ll be on my list to sue. Your company has a financial advantage over mine because you are violating federal law. That is clearly UNFAIR.
UPDATE: If you’re a supplement company, and you want to threaten me with legal action, watch this video first. The chickens will come home to roost!
A. If you feel your contract packager is responsible for following the 111’s and you do not have to you are 100% inaccurate and here is proof.
http://www.fda.gov/ICECI/EnforcementActions/WarningLetters/2015/ucm462745.htm
B. If you do have an FDA registration number and updated your status with the FDA, I will gladly remove you from this list.
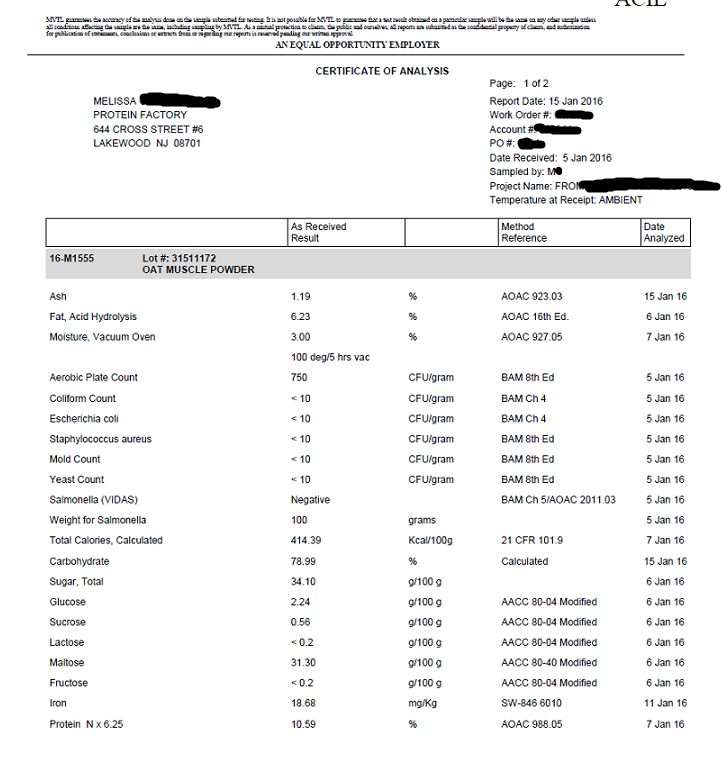
Now to my article…..The following is a list of supplement companies that have told me in one form or another that they may or may not be registered with the FDA, and some have flat out lied. I have put this list together so you can easily see who is breaking the law and who is not. In my opinion, it is also a good indicator of who is quality testing their products. I will update this list as more come in. Or if you have asked a supplement company for their FDA registration number and they responded with a “no” or possibly lied, please send it to me. [email protected]. I’ll give you a free T-shirt.
My other reason for doing this is because I feel it is unfair business practices to my company. My company follows the laws of this country and following the 111’s cost money, a lot of money. These companies that do not follow the 111’s have a financial advantage over my company and that is unfair. They can make more money, charge less for their products, and have a higher profit margin.
According to the Code of Federal Regulations 111’s for Dietary Supplements, all supplement companies MUST register with the FDA. This is not a choice or something voluntary. [clickToTweet tweet=”If you sell a dietary supplement (i.e., your name is on the label) you must register with the FDA.” quote=”If you sell a dietary supplement (i.e., your name is on the label) you must register with the FDA.”] Even if you do NOT physically make the supplement, you still must register with the FDA and comply with the CFR 111’s. Here’s the proof. (taken directly from the FDA’s website.
16.3 Q: Are dietary supplements considered “food” for purposes of the food facility registration requirement?
A: Under section 201(ff) of the FD&C Act (21 U.S.C. 321(ff)), a dietary supplement and a component of a dietary supplement is a “food.” Accordingly, a facility that manufactures/processes, packs, or holds a dietary supplement or a component of a dietary supplement is required to be registered as a food facility unless it qualifies for an exemption from registration.
Am I subject to the DS CGMP rule if I package, label, or distribute a dietary supplement manufactured by another firm?
Yes. The DS CGMP rule requires you to comply with those provisions directly applicable to the operations you perform.
For example, if you are a labeler, the DS CGMP rule:
Requires you to meet the requirement in 21 CFR 111.255 to establish a batch production record;
Requires you to comply with other applicable requirements, such as requirements for personnel, physical plant, and grounds, equipment and utensils, and holding operations;
Does not require you to comply with the requirement of 21 CFR 111.260(e) to include the identity and weight or measure of each component used, because you would be starting from packages that already had been filled rather than from individual components.
As another example, if you are a distributor who purchases a packaged and labeled dietary supplement and then holds the product in a warehouse for distribution to another physical location, the DS CGMP rule:
Requires you to comply with requirements for holding and distributing; and
Requires you to comply with other applicable requirements, such as requirements for personnel, the physical plant and grounds.
(21 CFR 111.1(a) and (a)(1); 72 FR 34752 at 34790 and 34886
Below you’ll see the company and sometimes the response they gave me when I simply asked them for their FDA registration number.
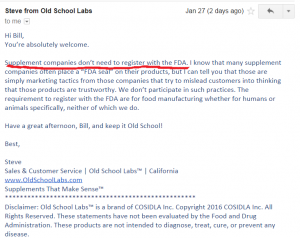
- Old School Labs. I emailed them asking them for their FDA registration number, and their response is that they do not need to register with the FDA.
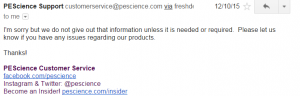
2. PEscience. Said they only give it out if needed..well yea I need it. And why is it a secret?
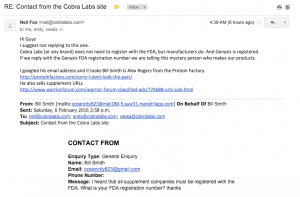
3. Cobra Labs. This one is hilarious. It looks like he meant to use this internally only but he emailed me as well. What is clearly obvious is that he does not think his company needs to register with the FDA because he physically does not manufacturer his supplements. The law clearly states that he is incorrect.
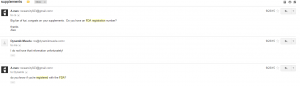
4. Dynamik. Said he couldn’t find it.
6. Athleanx. Lied and said supplement companies do not need to register with the FDA. I love how smug he sounds and is “clarifying” things for me. LOL
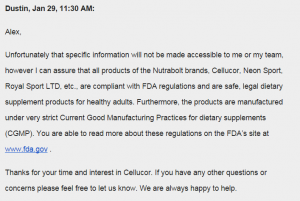
8. Cellucor. Said they have no clue I guess in one way or another.
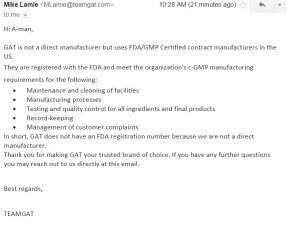
9. GAT. Flat out admitting that they do not have an FDA registration number.
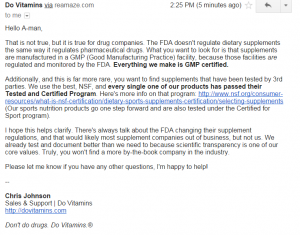
10. Dovitamins.
Here is a classic case of trying to bullshit you and dazzle you with footwork. 1st he says that it is not true that supplement companies don’t have to register with the FDA, Lie #1. Then he says dances and says what I really should look for is GMP and GMP certified, which is more bullshit. I love the last sentence where he says you won’t find another by the book company. Oh really asshole, you can’t even follow the FIRST STEP, which BTW is FREE. REGISTER WITH THE FDA, which is the LAW. Nice try, though. If I were a newb, you’d have me fooled with your magic show.
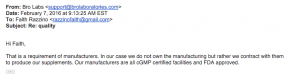
11. Brolabs. He is either ignorant or lying. Typical response..btw, no manufacturing facility is FDA approved. The FDA does not approve supplements and or manufacturing facilities..they just enforce the regulations.
More to come….don’t worry, if you own a supplement company and are NOT registered with the FDA you’re going to find yourself on this list. GET REGISTERED, it is the law!
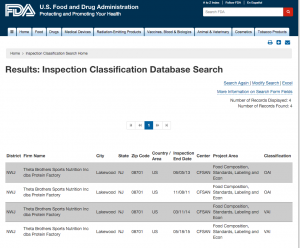
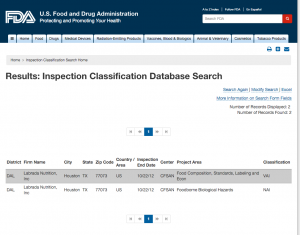
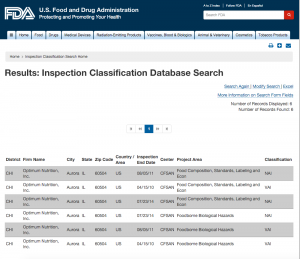
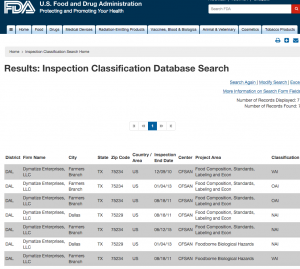
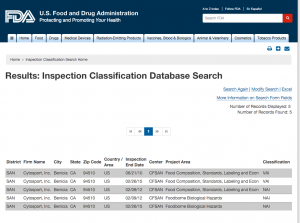
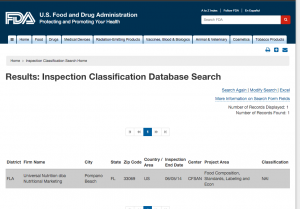
Here are companies that are registered with the FDA and you can see them by searching here. http://www.accessdata.fda.gov/scripts/inspsearch/index.cfm. By seeing a supplement company here, it means they are officially registered with the FDA and most likely are compliant with the 111’s, which ensure safe, clean, and true to label supplements.
1. Yours truly, Proteinfactory.com
2. Labrada
3. Optimum Nutrition
4. Dymatize
5. Cytosport
6. Universal
Alex Rogers is a supplement manufacturing expert. He has been formulating, consulting, & manufacturing dietary supplements since 1998. Alex invented protein customization in 1998 & was the first company to allow consumers to create their own protein blends. He helped create the first supplement to contain natural follistatin, invented whey protein with egg lecithin, & recently imported the world’s first 100% hydrolyzed whey.